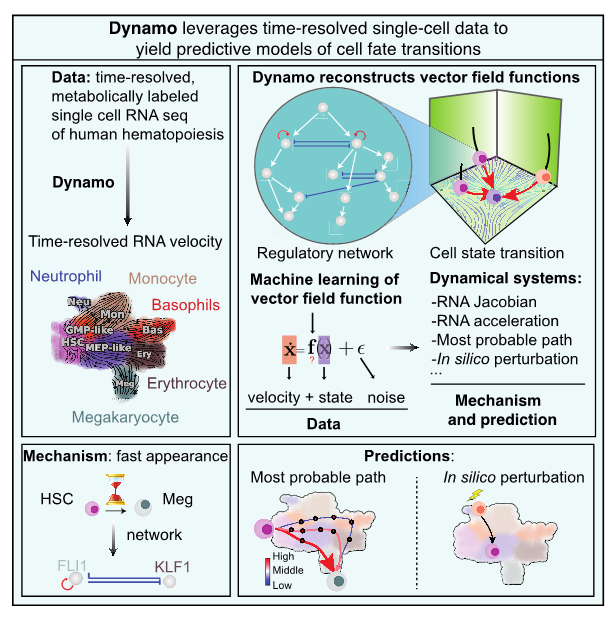
Message from the Dean
Ivet Bahar, PhD, founding chair of the Department of Computational and Systems Biology (CSB), will be resigning from her role effective December, 31, 2022 after 21 years of exceptional service. Ivet also holds the John K. Vries Chair in Computational Biology, has served as associate director of the University of Pittsburgh Drug Discovery Institute since 2010, and was elected to the National Academy of Sciences in 2020.
Ivet earned her MS in chemical engineering from Bogazici University, Istanbul, and her PhD in physical chemistry from Istanbul Technical University. She served for several years as professor of chemical engineering and founding director of the Polymer Research Center at Bogazici University before Pitt Med recruited her in 2001 to create a new Center for Computational Biology and Bioinformatics. The center’s success led to the formation in 2004 of the Department of Computational Biology (later renamed Computational and Systems Biology), one of the first such departments in the nation, with Ivet as founding chair.
In her nearly two decades at the helm of CSB, Ivet has firmly established a program of international impact. CSB’s highly interdisciplinary research faculty of both physical and biological science experts collaborate widely throughout the University, across the nation and around the world. The department also reflects Ivet’s longstanding commitment to developing the next generation of outstanding and diverse scientists in the field. In 2005, she co-founded the internationally acclaimed PhD program in computational biology, offered jointly with Carnegie Mellon University. In the past decade, she also established CSB’s Training and Experimentation in Computational Biology summer program for undergraduate students, funded by the National Science Foundation, and also supported the development of CompBio, one of the eight sites of the UPMC Hillman Cancer Center Academy for high school students. Both summer programs pull heavily from highly diverse academic, economic, and ethnic student populations.

Ivet signs the historical book during the inauguration of her elected membership of the National Academy of Science
Ivet is a pioneer in structural and computational biology, having developed widely-used elastic network models for examining the collective dynamics of proteins. In the past decade, she led the development and use of quantitative molecular and systems pharmacology models and methods, with applications to neurological disorders, cancer biology, and other diseases. More recently, she has contributed to understanding the molecular origin of multi-inflammatory (long) effects of COVID-19.
Ivet will be joining the State University of New York at Stony Brook as director of the Laufer Center for Physical and Quantitative Biology, a move that will also allow her to live closer to her children and grandchildren. Jeremy Berg, PhD, professor of computational and systems biology and associate vice chancellor for science strategy and planning, health sciences, is leading the search committee to find Ivet’s successor and will also serve as interim department chair.
Please join me in thanking Ivet for her vast contributions to our School of Medicine and wishing her the very best in her future.
Anantha Shekhar, MD, PhD
Senior Vice Chancellor for the Health Sciences
John and Gertrude Petersen Dean, School of Medicine
University of Pittsburgh.

 From Dean Shekhar:
From Dean Shekhar: