In the complex world inside our bodies, timing can be everything. A new study from Steven Smeal and Robin E.C. Lee reveals the timing of molecular signals can change how cells respond to their environment, with potential implications for cancer treatment and drug discovery.
How signals relay and process information is a critical gap in understanding cellular processes. To uncover these signaling processes, the research team focused on cytokines, small proteins that act as messengers between cells. They help regulate inflammation, immunity, and tissue repair, but they can also play contradictory roles. The same cytokine might promote cell death in one scenario and survival in another—even in the same cell type. In cancer, for example, certain cytokines can both suppress and encourage tumor growth.
“We started to wonder that in a body or even in a small clump of cells, is it possible that dynamic presentation of the stimuli might actually encode information to encourage cells to do various things, such as telling them to migrate or die?” said Robin Lee, associate professor.
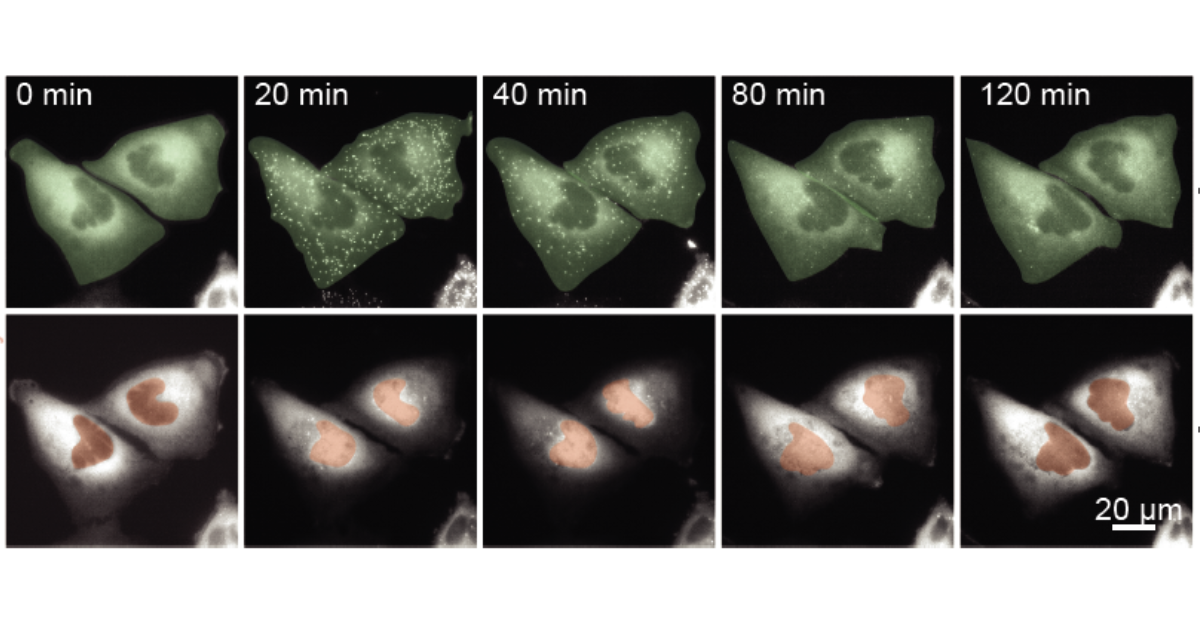
To test this idea, they used a custom, robot-controlled microfluidic system previously developed in the Lee lab. These tiny, chip-like devices let them expose cells to precise amounts of stimuli over time. They then used live-cell imaging to test the input-output encoding of IKK-NF-κB signals and compared cells exposed to a single cytokine pulse with pulses of varied duration. Surprisingly, cells responded more strongly to shorter, spaced-out pulses than to a single pulse.
They discovered that if cells are exposed to time-varying stimuli, it can regulate control points for cell fate decisions. To understand why, Smeal, PhD candidate and the paper’s first author, turned to computational modeling.
He began with a published model of NF-κB, a “master” transcription factor that controls hundreds of genes involved in inflammation, immunity, and cell survival or death. NF-κB normally sits in the cell’s cytoplasm, but when activated, it moves into the nucleus to switch genes on. The existing model didn’t have the correct structure to understand why smaller pulses were more effective, so Smeal adapted the model to better answer this question.
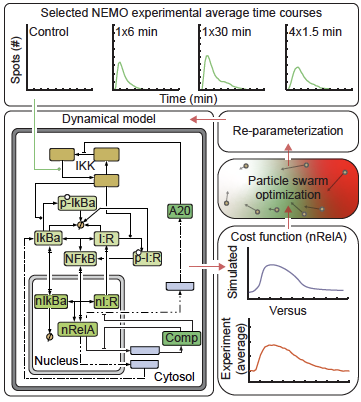
“I added two new mechanisms to the model,” Smeal said. “If NF-kB can bind to DNA, then it can shift the landscape of the DNA to the point where there are more binding sites and it’s sticking around in the nucleus longer.”
With the right architecture in place for their model, the team discovered that presenting small amounts of cytokine at different points in time can cause the biological system to reorganize genetic material in a way that it can access new genes.
The findings suggest that timing patterns in drug delivery could dramatically alter outcomes. Instead of focusing solely on giving the highest safe dose, therapies might work better with smaller, precisely timed pulses—an approach that could reduce side effects and improve effectiveness, especially in cancer treatment.
“There’s a whole other dimension that’s important to aspects of drug design,” said Lee. “It’s not just how much, but when.”
The research also opens the door to future experiments tracking multiple signaling pathways in the same cell, helping scientists predict and control cell fate more accurately.